41 what causes acid rain
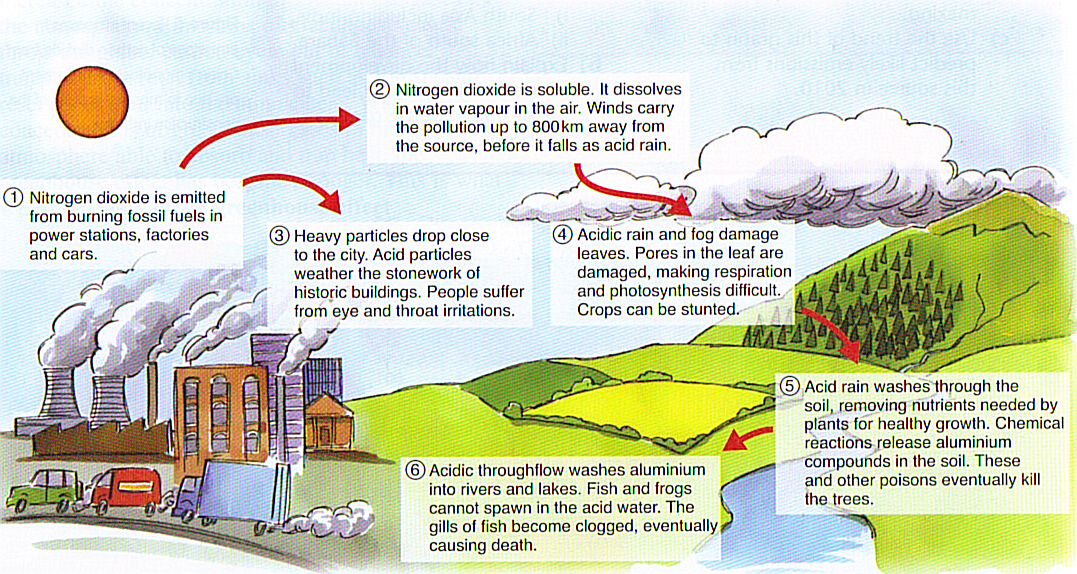
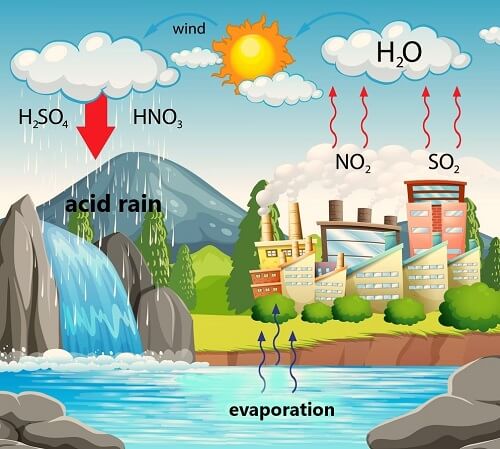
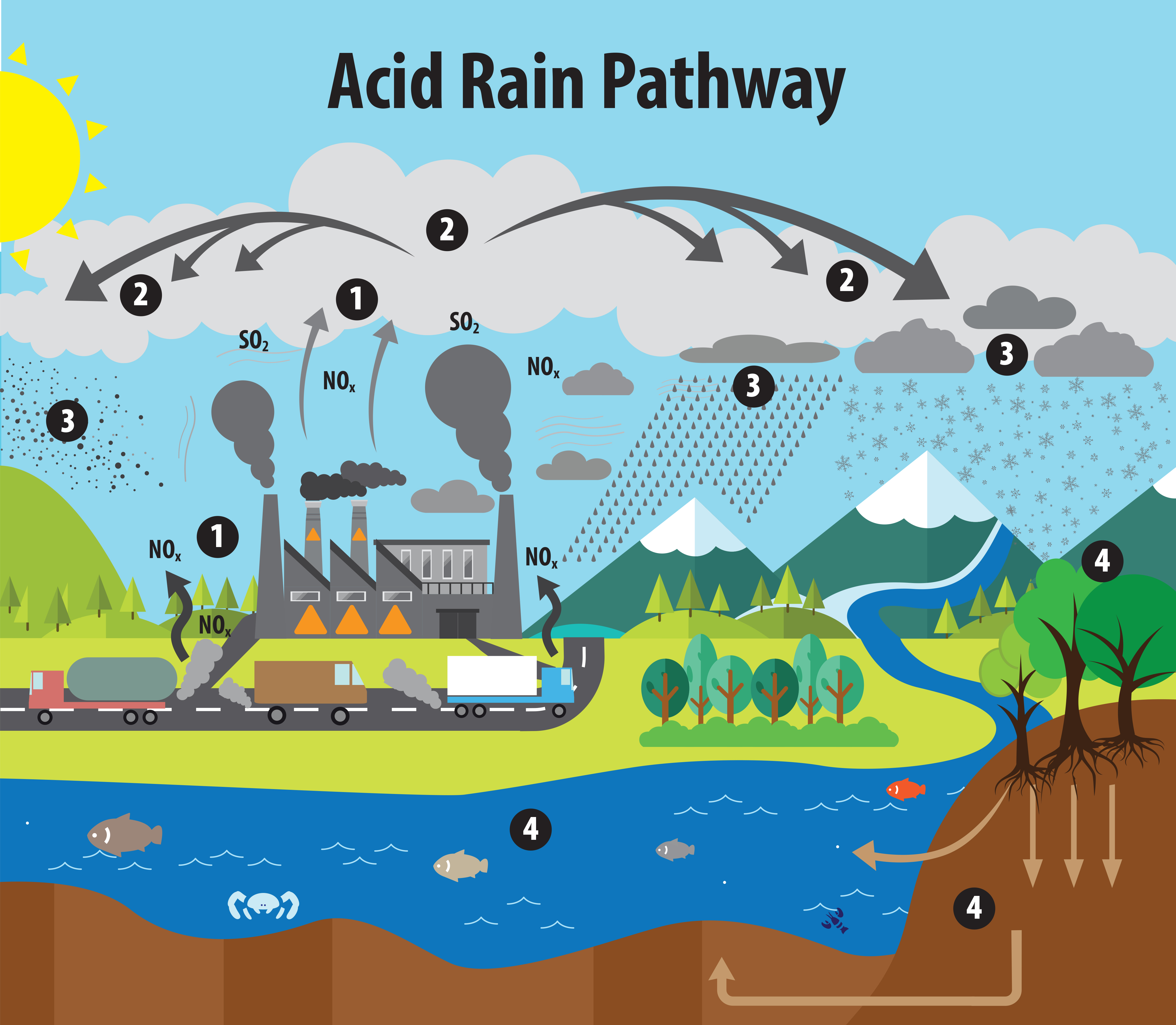
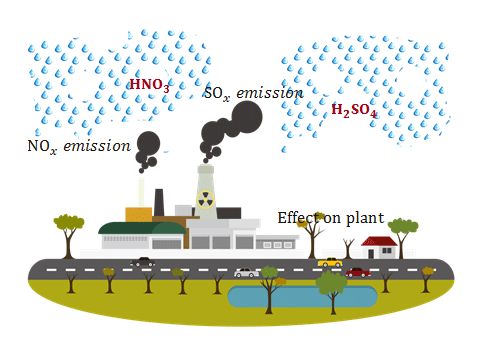
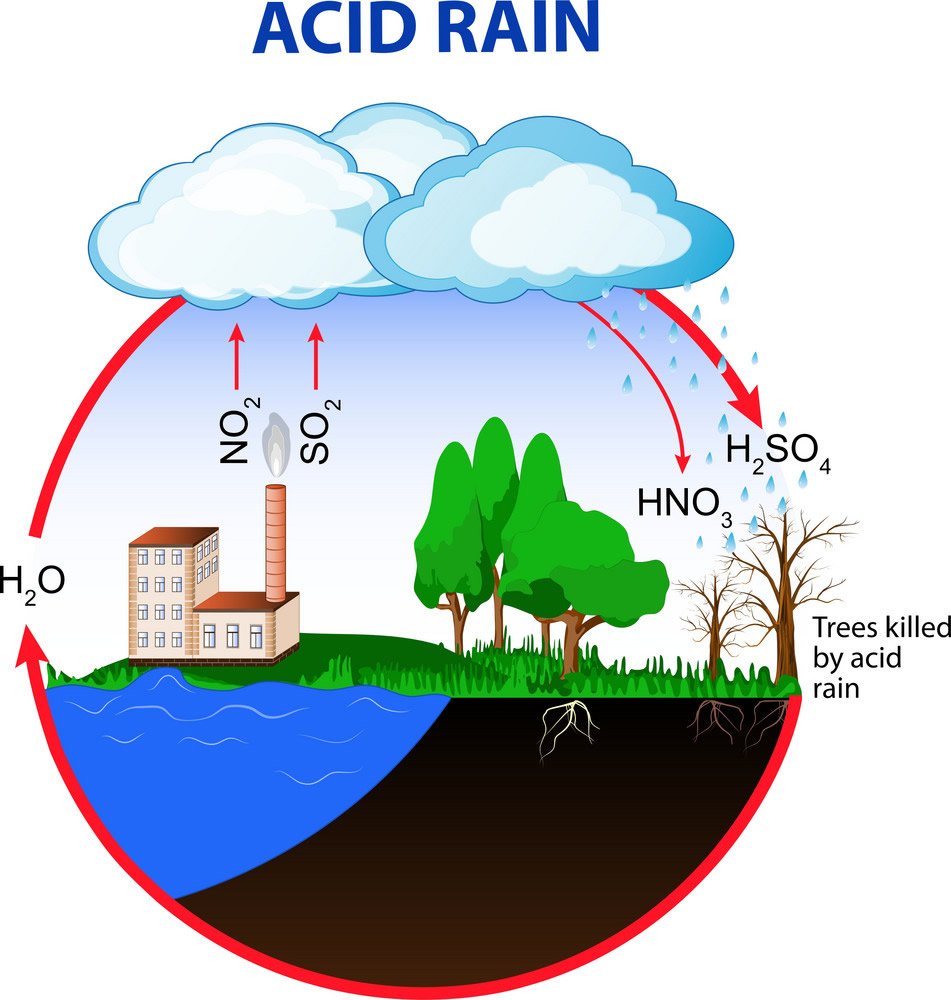
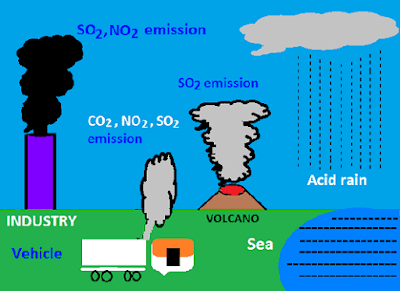
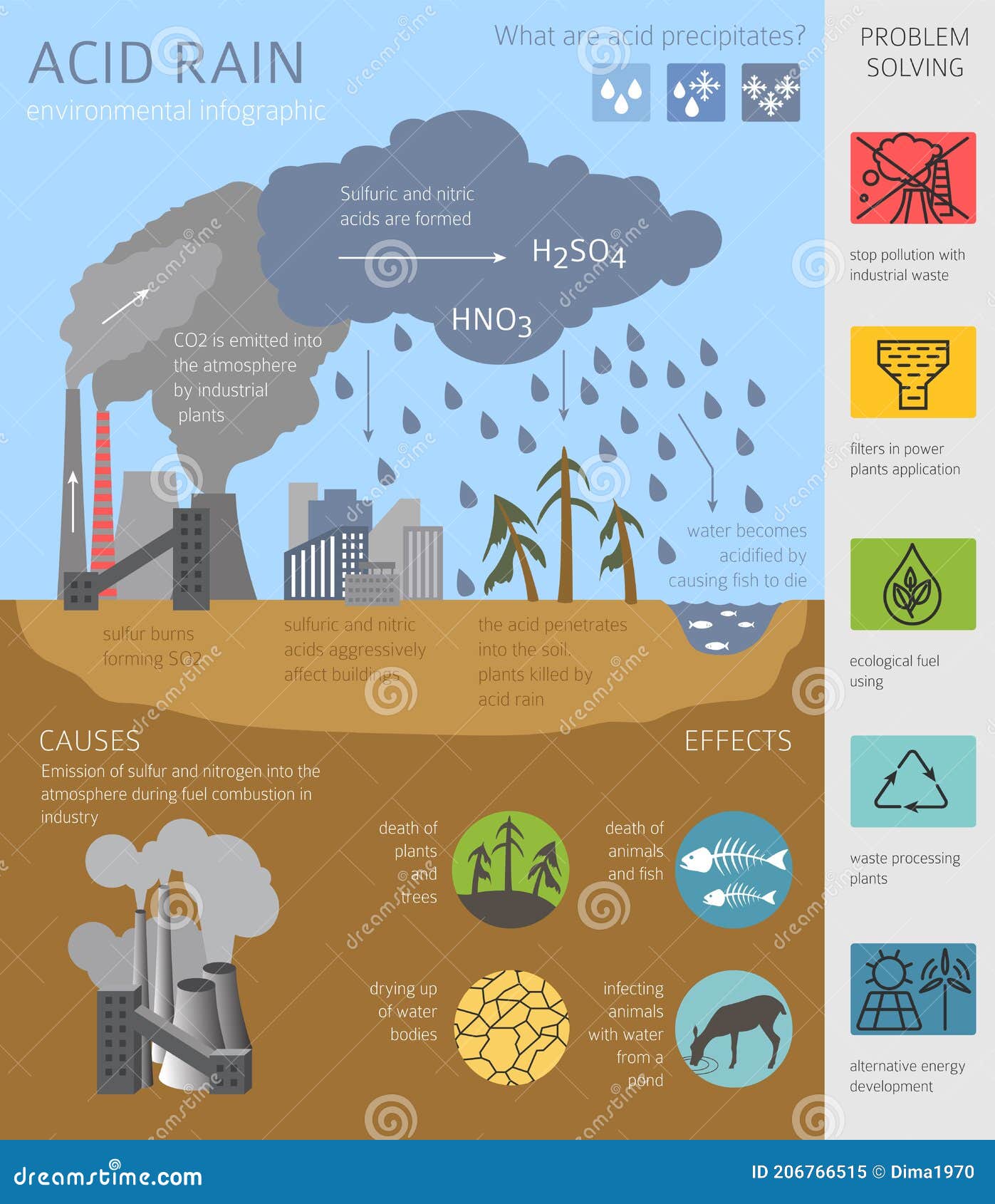
› acidrainAcid Rain - US EPA Feb 01, 2022 · The causes of acid rain, how acid rain affects our environment and our health, and what regulatory actions have been put in place to reduce the pollutants that cause acid rain. › acidrain › whatWhat is Acid Rain? - US EPA Acid rain results when sulfur dioxide (SO 2) and nitrogen oxides (NO X) are emitted into the atmosphere and transported by wind and air currents. The SO 2 and NO X react with water, oxygen and other chemicals to form sulfuric and nitric acids. These then mix with water and other materials before falling to the ground.
Acid rain: causes and effects - Canada.ca Acid rain occurs when acid-containing precipitation falls onto the earth's surface. Precipitation comes in the form of rain, snow, sleet, or hail. Precipitation collects acidic particles and gases and becomes acidic. These particles will have a pH level below 5.6. There are two types of deposition processes: wet and dry.

What causes acid rain
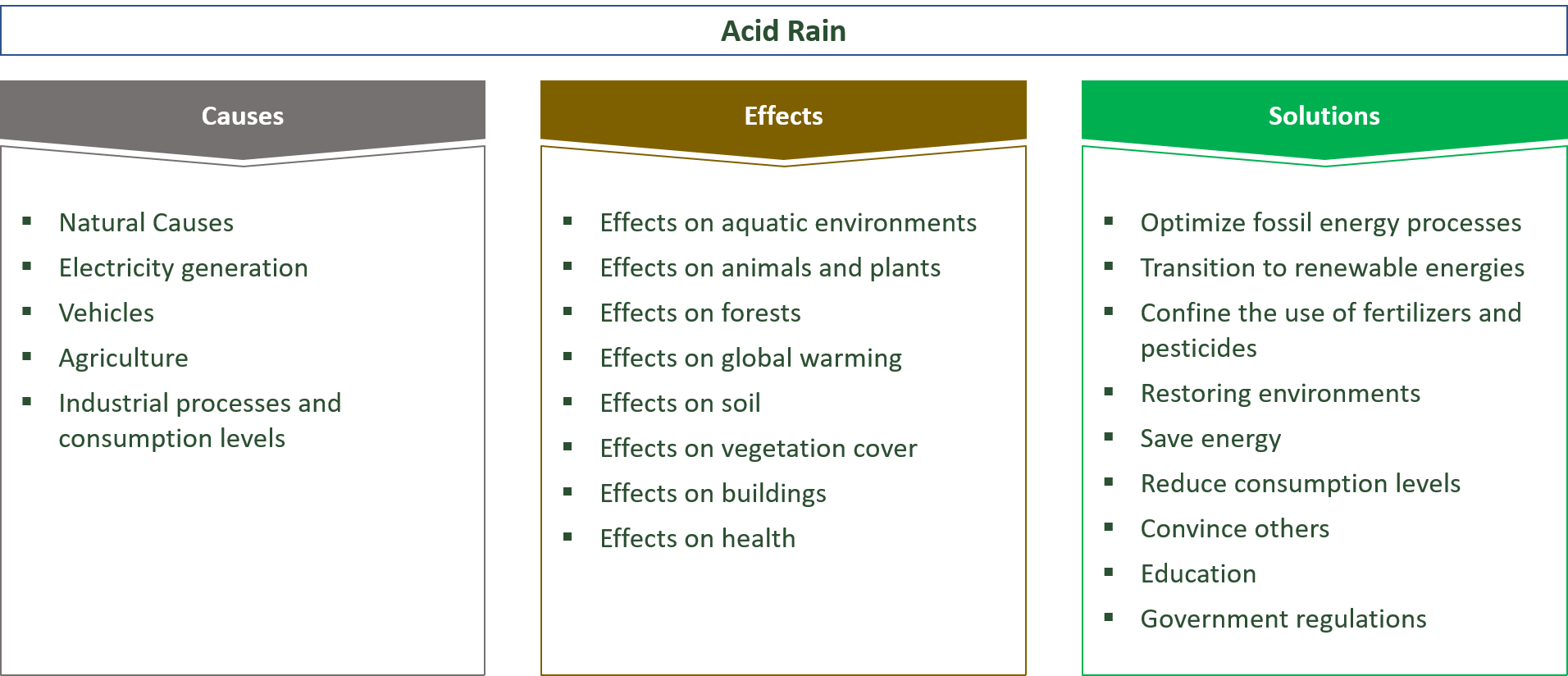
› 63065-acid-rainAcid Rain: Causes, Effects and Solutions | Live Science Mar 16, 2022 · Acid rain, or acid deposition, is a broad term that includes any form of precipitation that contains acidic components, such as sulfuric acid or nitric acid. It is caused by emissions of nitrogen oxide and sulfur dioxide, which react with water molecules in the atmosphere and produce acid rain. Acid rain has harmful effects on the environment, especially on aquatic animals and plants. It has also shown damaging effects on freshwaters, soils and insects. Stop Food Speculation! Acid Rain - Definition, Causes, Effects, Examples ... Acid rain is caused by a chemical reaction that begins when compounds such as sulphur dioxide and oxides of nitrogen are released into the air. These substances can rise very high up into the atmosphere, where they mix and react with water, oxygen, and other chemicals to form more acidic pollutants called acid rain.
What causes acid rain. What are Some Major Causes of Acid Rain (5 Steps) - HowFlux What are Some Major Causes of Acid Rain. Posted on January 22, 2016 February 2, 2018 by Editorial Team . You can understand acid rain as the rain that has got an acidic pH value that is less than seven. These types of rains are harmful for everyone and can make a huge loss to monuments, buildings, soil, plants and marine life as well. These ... Acid Rain: Causes, Effects and Solutions to Increase in ... Acid rain generally leads to weathering of buildings, corrosion of metals, and peeling of paints on surfaces. Erupting volcanoes contains some chemicals that can cause acid rain. Apart from this, the burning of fossil fuels, the running of factories and automobiles due to human activities are a few other reasons behind this activity. How does air pollution cause acid rain? - The science of air Acid rains are caused due to mixing of hazardous airborne pollutants into the atmosphere with water droplets and clouds. Acid rains, also known as acid precipitation, cause detrimental effects on the living organisms on the Earth. It can damage natural resources as well as animal health. What Are the Two Natural Causes of Acid Rain? - Brownfield ... Acid rain is rain that has an abnormally low pH level, indicating that it is particularly acidic. The release of hazardous particles into the atmosphere, such as sulfur dioxide (SO 2) and nitrogen oxide, causes acid rain (NO 2). These gases are removed from the air by falling into the soil or being absorbed by vegetation.
Effects of Acid Rain - US EPA Melting snow and heavy rain downpours can result in what is known as episodic acidification. Lakes that do not normally have a high level of acidity may temporarily experience effects of acid rain when the melting snow or downpour brings greater amounts of acidic deposition and the soil can't buffer it. Acid rain, explained | National Geographic Normal rain is slightly acidic, with a pH of 5.6, while acid rain generally has a pH between 4.2 and 4.4. Causes of acid rain. Rotting vegetation and erupting volcanoes release some chemicals that can cause acid rain, but most acid rain is a product of human activities. The biggest sources are coal-burning power plants, factories, and automobiles. › science › acid-rainacid rain | Definition, Causes, Effects, & Formulas | Britannica acid rain, also called acid precipitation or acid deposition, precipitation possessing a pH of about 5.2 or below primarily produced from the emission of sulfur dioxide (SO2) and nitrogen oxides (NOx; the combination of NO and NO2) from human activities, mostly the combustion of fossil fuels. In acid-sensitive landscapes, acid deposition can reduce the pH of surface waters and lower ... How to Prevent Acid Rain - Green Coast The main source of acid rain is human-caused burning of fossil fuels. Sulfur is a contaminant, principally of coal, oil, and diesel fuel, that is released when they're burned in power plants, homes, some cars, or construction vehicles. Once in air, sulfur combines with oxygen to form sulfur dioxide.
Primary Causes of Acid Rain | Earth Eclipse Primary Causes of Acid Rain Acid rain is formed by elevated levels of sulfur and nitric acids in the atmospheres that accumulate as a result of Nitrogen oxides (NOx) and Sulfur dioxides (SO2) emissions. Acid rain is a mix of atmospheric water molecules and dry depositions of Sulfur dioxides and Nitrogen oxides emitted from industries and vehicles. The Causes of Acid Rain | Cuarl The biggest cause of acid rain are sulphates, an air pollutant that come primarily from coal-fuelled power plants and the burning of peat. Acid rain is caused by many other pollutants as well, which have varying effects on waters, fish and plant life. The most common cause of acid rain is the pollution caused by fossil fuels. What Causes Acid Rain: Causes, Impact & Solutions - Utopia Acid rain is caused primarily by sulfur dioxide (SO2) and nitrogen oxide (NOx) emissions. When these gases react with the moisture in the atmosphere, sulfuric and nitric acids form. Wind may transport these acid particles over long distances before they come down as wet or dry deposition. Acid Rain - Definition, Causes, Effects, Examples ... Acid rain is caused by a chemical reaction that begins when compounds such as sulphur dioxide and oxides of nitrogen are released into the air. These substances can rise very high up into the atmosphere, where they mix and react with water, oxygen, and other chemicals to form more acidic pollutants called acid rain.
It is caused by emissions of nitrogen oxide and sulfur dioxide, which react with water molecules in the atmosphere and produce acid rain. Acid rain has harmful effects on the environment, especially on aquatic animals and plants. It has also shown damaging effects on freshwaters, soils and insects. Stop Food Speculation!
› 63065-acid-rainAcid Rain: Causes, Effects and Solutions | Live Science Mar 16, 2022 · Acid rain, or acid deposition, is a broad term that includes any form of precipitation that contains acidic components, such as sulfuric acid or nitric acid.











/cloudfront-us-east-1.images.arcpublishing.com/gray/6FU2D4CTCBFOZN2MZEIJZXYQUE.png)








0 Response to "41 what causes acid rain"
Post a Comment